Assignment
Short Answer Quest
1. A 25 year old woman complains of severe pain in abdomen, photosensitivity, constipation, abnormal behavior and seizures. On examination Faster heart rate and higher blood pressure. Biochemical investigations showed high porphobilinogen (PBG).
a. What is the probable diagnosis?
b. Why did the patient have these symptoms?
c. What are the biochemical investigations required?
2. Explain why von-gierke’s disease presents with hyperuricemia.
3. What is lactic acidosis? Write a note on Cori’s Cycle.
4. A 36 Years old woman was brought to emergency room complaining of dizziness. She had missed her dinner and breakfast. She had also started following a rigorous exercise regime to lose weight. Plasma glucose = 43 mg/dL.
a. Give the probable Diagnosis
b. What measures must be taken for managing the condition?
c. Mention two other causes for the above condition?
d. What is the normal fasting plasma glucose concentration?
e. What advice should be given to the patient?
5. TCA is an amphibolic pathway. Giving examples defend how this statement is true.
6. Diet prescription for Diabetes mellitus and Atherosclerosis.
7. Describe the products of HMP shunt pathways and its significance.
8. Mucopolysachharides
9. Dietary fibers and their clinical significance
10. What are Glycosaminoglycans, Give their Functions and Disorders11. Describe Active Form, RDA, Biochemical Functions and Deficiency Manifestations of Pyridoxine
12. Mention Five markers in cardiac diseases and their patterns in myocardial infarction
13. Define Electrophoresis its Types , Principle and Clinical Applications
14. Thalassemia :- biochemical defect, clinical features, biochemical tests
15. Describe doctor Patient relationship
16. Classify the Immunoglobulins. Give Structure of immunoglobulins. Add Note On Clinical
Applications of immunoglobulins
17. Explain in detail about the Role of the Kidney in Acid–acid-base balance
18. CSF in health and diseases
19. Discuss classification and etiology of fatty liver and add a note on Lipotropic factor
20. Mention any five Diagnostic and therapeutic uses of enzymes
21.Describe Fatty liver with respect to causes and biochemical basis.
22.What are tumor markers and give 5 examples in diagnosis of different diseases.
23.Enumerate the sources of Oxidative radicals. Describe the biochemical actions of
four antioxidants.
24. Role of CSF analysis in various diseases.
25.Explain Mucopolysaccharides types function and disorders
26.Describe Kwasiorkar and nutritional marasmus
27.Describe Collagen-types, structure, functions and names of two genetic diseases.
28.Explain Immunoglobulins structure, function and applications
29.Describe Inhibitor and uncouplers of ETC
30. ADA criteria for Diabetes mellitus and Gestational Diabetes Mellitus
31. Describe Vitamin B12 deficiency and folate trap. Add a note on homocysteinemia.
32. Molecular, clinical, and diagnostic differences between sickle cell anemia and thalassemia
33. Enumerate various Molecular diagnostic Techniques and its applications
34. Hyperammonemia causes, biochemical basis and lab diagnosis
35. Neonatal and congenital hyperbilirubinemia.
36. Dehydration; Types and biochemical changes in Dehydration
37.Hemoglobin Catabolism and Diagnosis of various types of jaundice
38. Biochemical functions of Iron with its associated clinical disorders.
39. Describe metabolism of alcohol with Biochemical changes and effects of chronic alcoholism.
40. Thyroid disorders and function tests
41.Explain any 5 tumor markers and conditions associated with it.
42.Define & Classify Lipids based on Functions. Note on lipidosis
43.Immunoglobulin :- Types, Functions and Disorders
44.Principle and Clinical applications of Chromatography. Add a note on HPLC
45.Define Oxidative phosphorylation. Enumerate inhibitors & Uncouplers of Oxidative phosphorylation.
Add note on Chemiosmotic Theory
46.Types of Enzyme Inhibitions with Suitable Examples? add a note on therapeutic uses of enzymes
47.Structure and Functions of Cholesterol
48.Enumerate plasma proteins and Write a note on the role of protein in water regulation / balance
49.Therapeutic and diagnostic uses of Enzymes
50.Enumerate Biologically Important Polypeptides. Explain them in relation to its clinical importance in practice.
51.Define clearance test and discuss various clearance tests and their clinical significance
52.Define xenobiotic metabolism. Explain Phase I reactions.
53. PEM
54. Immunoglobulin, Structure, types and function
55. Describe tumor mar.kers and give five examples in diagnosis of different diseases.
56. Prostaglandin’s, classification & uses in medicine.
57. Cholesterol biosynthesis & its regulatory step.
58. Phase II detoxification reactions
59. Metabolic fate of Acetyl CoA
60. Inhibitor & uncoupler’s of ETC
61. Ketogenesis and Ketolysis
62. Regulation of blood glucose level
63. How is HMP shunt related to Steroid Hormone synthesis?
64. Biochemical rationale of ketogenic diet in weight loss.
65. Dietary modifications in a case of Diabetes Mellitus Type2.
66. Differentiation of different types of jaundice using enzymes.
67. Glycated hemoglobin and its role in Diabetes Mellitus
68. Compare the functions of various types of Immunoglobulins
69. Enumerate Type II conjugation reactions with examples
70. Highlight the differences between Kwashiorkor and Marasmus.
71. Role of Glut4 transporter in health and disease.
72. Role of short chain fatty acids in the body.
73. Five diagnostic and therapeutic uses of Enzymes.
74. Dietary modifications in a case of Diabetes Mellitus Type2.
75. Tumor markers with suitable example.
76. Dietary fibers and their clinical utility.
77. Role of CSF analysis in diseases.
78. Immunoglobulins: Structure and function
79. Lipoproteins: Add a note on Hype lipoproteins. (2+3)
80. Define free radicals & name four Reactive oxygen species and damage caused by them.
81.Structural organization of proteins
82.Salvage pathway for nucleotide synthesis
83.Mucosal block theory
84.Dehydration, Types and Biochemical changes
85.Recombinant DNA technology and it’s Uses.
86.ELISA
87.Heme catabolism and differential diagram of Jaundice by LFt’s
88.Folate trap
89.Draw the purine ring; write the sources of carbon and Nitrogen atoms of the ring.
90.Genetic code and it’s properties
Long Answer Question (LAQ)
1. Discuss the synthesis and breakdown of glycogen. Write a note on its regulation. Add a note on glycogen storage disorders
2. Discuss how blood glucose is regulated.
3. Discuss the regulatory role of PFK1, glucokinase and glycogen phosphorylase in carbohydrate metabolism4. Describe the process of gluconeogenesis
5.Describe the Electron Transport chain under the following headings,
- i. Definition
- ii. StructuralOrganisation of Components of Complexes with Diagram,
- iii. Reactions of Each Complex With Diagram.
- iv. Inhibitors of Each complex
6. Describe Enzymes under following headings
- i. Active site of the Enzyme,
- ii. Mechanism of enzyme action
- iii. Diagnostic uses of enzymes
7. Describe Diabetes Mellitus under the headings -
- i. Definition & Types
- ii. Biochemical basis for clinical features & complications
- iii. ADA Criteria for Laboratory investigations for diagnosis and long-term case management of Diabetes Mellitus
8. Describe cholesterol under following headings
- i. Regulation of rate-limiting step of cholesterol synthesis & its significance
- ii. Specialized products synthesized from cholesterol & their Functions.
- iii. Add a note on disorders associated with those products.
9. Write Active forms, Sources, RDA, Mention FOUR Biochemical Functions & Deficiency manifestations of Vitamin A. Explain the process of Walds Visual Cycle in Vision
10.Describe lipoproteins under the following headings
- i. Explain HDL transport mechanism and why it is a good Cholesterol
- ii. Explain LDL transport mechanism and why it is a bad Cholesterol
- iii. Hyperlipoproteinemias classification & clinical significance
11.Explain the gluconeogenesis in details under following headings -
- i. Metabolic pathway with all enzymes
- ii. Regulation in fasting state of metabolism
12. Describe the metabolism of lipoproteins with respect to
- i. formation & transport
- ii. Hyper lipoproteinemias
- iii. Relations of LDL with atherosclerosis
13. i. Describe the process of Translation in eukaryotes
ii. Mention any five post translational modifications
iii. Inhibitors of translation
14. i. Explain the role of blood buffers in acid-base balance
ii. Role of kidney in maintaining pH
iii. Write a note on Acidosis and Alkalosis
15. a) Discuss the Gluconeogenesis under following headings (1+2+7)
i. What is Gluconeogenesis?
ii. Substrates for gluconeogenesis.
iii. Explain the pathway, regulation and bioenergetics of gluconeogenesis from
propionyl CoA?
16. Describe metabolism of lipoproteins with respect to:
i. Formation and transport
ii. Hyper lipoproteinemia
iii. Role of LDL in atherosclerosis
17. A 14 year old girl, while on a school trip to the mountains got exposed to a snowstorm. Within
a couple of hours, she started passing yellowish urine and felt extremely weak. She had no
history of similar episode in the past. On examination:
Urine for bilirubin: Positive
Icterus: Positive
Peripheral blood smear: Abnormally crescent shaped RBC’s
Haemoglobin: 10.3gm/dl
i. What is the probable diagnosis?
ii. What defect is present in the structure of Haemoglobin?
iii. What will be the expected Liver Function Test report in this case of Serum Total
Bilirubin, Direct and Indirect Bilirubin, AST, ALT and ALP?
18. Regarding fatty acid oxidation
i. Enumerate types of fatty acid oxidation
ii. Define β-oxidation of fatty acid.
iii. Describe the β-oxidation of palmitic acid, including the energetics involved.
19. Discuss the metabolism of ketone bodies with suitable diagram under; (6+2+2)
i. Ketogenesis and ketolysis.
ii. How you differentiate ketosis of DKA from starvation?
iii. Clinical significance of ketone bodies.
20. Describe fatty acid oxidation (2+1+7)
i. Enumerate different types of fatty acid oxidation.
ii. Define β-oxidation.
iii. Describe the β-oxidation of palmitic acid (C16) including the energetics involved.
21. Give the answer of following.
i.Explain the role of blood buffer in acid base balance (2+3+2.5+2.5)
ii. Role of kidney in maintaining PH of blood.
iii. Biochemical
changes in metabolic Acidosis and Alkalosis.
22.Regarding amino acid methionine. (3+4+3)
i.Enumerate the functions of methionine in the body
ii.Describe in detail about the metabolism of methionine
iii.Add a brief note on a disorders associated with methionine metabolism
Case summary
1. A 30-year-old working woman had been in the habit of either not eating or eating freeze-stored food for
over a year. She developed the symptoms of spongy, sore & bleeding gums. She used to have frequent
common colds and respiratory tract infections. Also observed delayed wound healing and petechial
hemorrhage & swollen joints.
- i. What is the probable diagnosis?
- ii. What are the sources of the Deficiency biomolecule?
- iii. What is the biochemical basis of disease?
- iv. What is the cause of anemia?
2. An 8-year-old girl from an endemic malaria area who had splenomegaly was investigated for routine
hematology, which revealed low hemoglobin of 7 gm%. Peripheral smear revealed - crescent-shaped
RBCs. She had no history of malaria attacks.
- i. What is the probable diagnosis?
- ii. What is the Biochemical basis of diseases?
- iii. Name the biochemical investigations that can be done to confirm the diagnosis
- iv. Reason out why these patients show resistance to malaria.
3. A 2-year-old boy presents with developmental delay, aggressive behavior, and frequent episodes of
self-mutilation, including biting of lips and fingers. The mother reports that the child's diaper often
contains orange sand-like crystals. Laboratory tests reveal markedly elevated serum uric acid levels.
Based on this clinical case, answer the following:
- i. What is the most probable diagnosis?
- ii. Name the defective enzyme and the gene involved.
- iii. Describe the metabolic pathway affected and the reason for hyperuricemia.
- iv. Mention one specific clinical feature that distinguishes this condition from other hyperuricemic disorders.
4. A 4 years old boy was brought by parents to the OPD with complaints of severe fatigue, shortness of
breath after he had treatment for malaria last week. For 2-3 days, he is passing dark colored urine.
Pediatrician noted mild jaundice and an increased heart rate. The paediatrician suspects a disease for
which he asked for similar complaints in parents or in close relatives.
- i. What might be the diagnosis?
- ii. Which blood tests would be prescribed & why?
- iii. Explain the biochemical basis for the disease?
5. A 55 years old male known case of Diabetes Mellitus on insulin was admitted to the ICU in
semiconscious state. Examination revealed Kussmaul’s breathing with a fruity smell. On
investigations, Random blood glucose was 700mg/dl. - Urine: ketones ++, Blood pH 7.1,
HCO3 = 19 mmol/L, pCO2=36 mmHg
- i. What is the probable diagnosis?
- ii. What are the reasons for each abnormal lab report?
- iii. What is the biochemical basis of this condition?
6. After 6 hours of birth a premature infant was found to have cyanosis, grunting, poor feeding,
tachypnea (>60 breaths/min) and intercostal retractions. On bronchography airless, collapsed alveoli
were observed against air-filled bronchi along with decreased lung volumes.
- i. What is the probable diagnosis?
- ii. What is the biochemical basis of respiratory distress?
- iii. Which biochemical test is used for diagnosis of this disease?
- iv. What is the treatment?
7. A 55-year-old male known case of Diabetes Mellitus on insulin was admitted to the
ICU in semi unconscious state. Examination revealed Kussmaul’s breathing with a
fruity smell. On investigations, Random blood glucose was 700mg/dl.-Urine: ketones++,
- i. What is the probable diagnosis?
- ii. What are the reasons for the abnormal lab report?
- iii. What is the biochemical basis of this condition?
8. The 7-year-old boy complains of pain abdomen and loose stools and has no fever.
On further history taking symptoms usually follow after taking milk. On examination,
no significant abnormality was detected.
- i. What is the probable diagnosis?
- ii. What is the biochemical basis of these symptoms?
- iii. What are the tests to be done on this patient?
- iv. what is the treatment?
9.A 20-day-old infant was brought to the OPD with complaints of frequent convulsions
and vomiting. On further evaluation, it was noted that the infants urine smelled like
burnt sugar. His urinary Rothera test was positive.
- i. What is the probable diagnosis?
- ii. Write the biochemical basis of this condition
- iii. How to manage this case?
10. A 45-year-old woman on steroids presents to the clinic with a history of progressive weight
gain, specifically in her face and trunk, over this year. She also reports easy bruising,
developing purple stretch marks on her abdomen and thighs. She is experiencing muscle
weakness and increased fatigue. She also mentions experiencing mood swings, difficulty
sleeping and irregular menstrual cycles.
- i.What is the diagnosis of this condition?
- ii.Explain the biochemical basis for the symptoms.
- iii.Which biochemical tests can be prescribed to support and confirm diagnosis of the disease?
11. a) Diagnostic and therapeutic uses of enzyme
b) ADA criteria for diagnosis of Diabetes Mellitus and Gestational Diabetics
c) A 25-year-old male on prolong fast (› 3days) complained of headache& fatigue. His breath
has fruity odour. Blood glucose is 60 mg/dl. Ketones are positive. ABG: shows metabolic
acidosis.
i. What is the biochemical basis of Ketosis? (1)
ii. Differentiate between starvation Ketosis & diabetic ketoacidosis. (1)
iii. Explain the role of lipolysis & fatty acid oxidation is ketogenesis. (2)
iv. What is the cause of fruity odour & names of ketone bodies?
12. a) Compare active and passive immunity
b) Role of oncogenes in tumour development and progression
c) Factors regulating fluidity of cell membrane
13. a) A 4-week-old baby is being seen by the pediatrician because of frequent vomiting after
meals and tenderness in the abdomen. Upon examination, the physician noted an enlarged
liver and a hint of cataract formation in both of the child’s eyes. A urine dipstick test for a
reducing sugar gave a positive result. Blood glucose levels were slightly below normal.
i) What is probable diagnosis?
ii) What is biochemical cause of Cataract.
ii) What dietary restrictions are advised?
b) Role of oncogenes in tumour development and progression
c) Phospholipids and their functions.
14. A mother notices mousy odour coming from her five-year-old child. The child had seizures and has developed a hypopigmented patch on skin. On consulting a paediatrician records a developmental delay. (2+3)
i. What is the provisional diagnosis and why?
ii. What treatment option is provided to the mother? On lab investigation, which analyte will be raised in blood?15. A 45-year-old female presented with itching and yellowish discoloration of skin. She also reports passage of clay colored stool and abdominal pain. (1.5+3.5)
i) What is the provisional diagnosis and why?
ii) What is direct and indirect bilirubin? On lab investigation, which enzymes would be raised?
16.An alcoholic ends up in emergency with confused state. He is showing amnesia. He is diagnosed as Wernicke's encephalopathy, a neurological condition, and Korsakoff's psychosis, a neuropsychiatric disorder.
i. Which nutrient deficiency is the reason for the state?
ii. What is active form of the above-mentioned nutrient?Reasoning
1. Ethanol used as antidote in methanol poisoning
2. Glucose 6 Phosphatase deficiency associated with hypoglycaemia and hepatomegaly
3. Aspirin reduces risk of thrombotic event
4. HbA1C is reliable indicator of long term treating glycemic control
5. RBCs rely on glycolysis for energy
6. Cataract in Galactosemia
7. Reason for Menkes and Wilson’s disorder
8. Reason for Xeroderma pigmentosum
9. Mention the drug used in treatment of gout and its biochemical basis of its mechanism of action
10. Steatorrhea causes fat soluble vitamin deficiency.
11. Reasons to choose between mGFR and eGFR.
12. use of chromatography for diagnosis of Inborn errors of metabolism causing aminoaciduria.
13. Why HBA1c is used for monitoring of glycaemic status in diabetes mellitus?
14. What is use of Lecithin by sphingomyelin ratio in respiratory distress syndrome ?
15. Mitochondria is called powerhouse of the cell
16. Fatty acids cannot be converted into glucose.
17. Starch can be digested by humans but cellulose cannot..
18. Ethanol is used as antidote in methanol poisoning.
19. Dietary fibre increased consumption reduces serum cholesterol levels.
20. Use of Methotrexate as anticancer drug.
21. Physiological uncouplers of ETC and its role in Kernicterus?
22. Bloating and flatulence in lactose intolerance.
23. Dietary fibre consumption reduces blood cholesterol levels
24. How do Vitamins act an anti-oxidants?
25. Why those suffering from diabetes are likely to develop cataract ?
26. Linoleic and linolenic acids are crucial in diet.
27. Use of Methotrexate as anticancer drug.
28. Hypoglycemia is more dangerous than Hyperglycemia.
29. Anaplerotic reactions replenishes the Intermediates of TCA Cycle
30. Hemolysis should be avoided in blood sample for enzyme assay.
31.Why is Macrocytic RBC a feature of Vitamin B12 deficiency?
32.Compensatory mechanism in a case of respiratory acidosis.
33.Elaborate on the role of sodium in ORS.
34.Why ALP is high in growing children
35.Population having staple diet of maize may have pellagra
36.Steatorrhea can cause deficiency of fat soluble vitamins
37.
AETCOM
1. Enumerate and describe professional qualities and roles of a physician.
2. Describe and discuss the role of a physician in health care system.
3. Roles of IMG as a Physician
4. How would you maintain patient confidentiality when dealing with a colleagues’s family?
5. Enumerate and describe professional qualities and roles of a Physician. What is
importance of lifelong learning as an important part of physician growth?
6.Describe and discuss the role of a physician in health care system and responsibilities to
society and the community that she/he serves.Daily based Assignment For Biochemistry
Long Questions
- Define mucopolysaccharides and discuss their role as structural elements in the human body with examples.
- Define and classify carbohydrate with examples of each and their clinical/biological significance.
Short Answer Questions
- Discuss properties and biological significance of disaccharides, with examples.
- Define and discuss the importance of glycemic index giving examples of foods with high and low glycemic index.
Question Bank
Practical Biochemistry
Basic Biochemistry
Cell, in biology, the basic membrane-bound unit that contains the fundamental molecules of life and of which all living things are composed. A single cell is often a complete organism in itself, such as a bacterium or yeast. Other cells acquire specialized functions as they mature. These cells cooperate with other specialized cells and become the building blocks of large multicellular organisms, such as humans and other animals. Although cells are much larger than atoms, they are still very small. The smallest known cells are a group of tiny bacteria called mycoplasmas; some of these single-celled organisms are spheres as small as 0.2 μm in diameter (1μm = about 0.000039 inch), with a total mass of 10−14 gram—equal to that of 8,000,000,000 hydrogen atoms. Cells of humans typically have a mass 400,000 times larger than the mass of a single mycoplasma bacterium, but even human cells are only about 20 μm across. It would require a sheet of about 10,000 human cells to cover the head of a pin, and each human organism is composed of more than 30,000,000,000,000 cells.
Chemistry of Biomolecules
Chemistry of Biomolecules focuses on the chemistry underpinning the biological roles of proteins, carbohydrates, nucleic and lipids. You will learn about the link between structure and function of these molecules at a chemical level within a biological context. Overview lectures will bring together this knowledge and apply it to key chemical process relevant to life: respiration, disorders treatment and signalling.
Carbohydrates
Carbohydrates are chemically defined as polyhydroxy aldehydes or ketones or compounds which produce them on hydrolysis. In layman’s terms, we acknowledge carbohydrates as sugars or substances that taste sweet. They are collectively called as saccharides (Greek: sakcharon = sugar). Depending on the number of constituting sugar units obtained upon hydrolysis, they are classified as monosaccharides (1 unit), oligosaccharides (2-10 units) and polysaccharides (more than 10 units). They have multiple functions’ viz. they’re the most abundant dietary source of energy; they are structurally very important for many living organisms as they form a major structural component, e.g. cellulose is an important structural fibre for plants.
Lipids
Lipids are organic substances that are insoluble in water, soluble in organic solvents, are related to fatty acids and are utilized by the living cell. They include fats, waxes, sterols, fat-soluble vitamins, mono-, di- or triglycerides, phospholipids, etc. Unlike carbohydrates, proteins, and nucleic acids, lipids are not polymeric molecules. Lipids play a great role in the cellular structure and are the chief source of energy.
Proteins
Proteins are another class of indispensable biomolecules, which make up around 50per cent of the cellular dry weight. Proteins are polymers of amino acids arranged in the form of polypeptide chains. The structure of proteins is classified as primary, secondary, tertiary and quaternary in some cases. These structures are based on the level of complexity of the folding of a polypeptide chain. Proteins play both structural and dynamic roles. Myosin is the protein that allows movement by contraction of muscles. Most enzymes are proteinaceous in nature.
Nucleic Acids
Nucleic acids refer to the genetic material found in the cell that carries all the hereditary information from parents to progeny. There are two types of nucleic acids namely, deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). The main function of nucleic acid is the transfer of genetic information and synthesis of proteins by processes known as translation and transcription. The monomeric unit of nucleic acids is known as nucleotide and is composed of a nitrogenous base, pentose sugar, and phosphate. The nucleotides are linked by a 3’ and 5’ phosphodiester bond. The nitrogen base attached to the pentose sugar makes the nucleotide distinct. There are 4 major nitrogenous bases found in DNA: adenine, guanine, cytosine, and thymine. In RNA, thymine is replaced by uracil. The DNA structure is described as a double-helix or double-helical structure which is formed by hydrogen bonding between the bases of two antiparallel polynucleotide chains. Overall, the DNA structure looks similar to a twisted ladder.
Enzymes
The biological processes that occur within all living organisms are chemical reactions, and most are regulated by enzymes. Without enzymes, many of these reactions would not take place at a perceptible rate. Enzymes catalyze all aspects of cell metabolism. This includes the digestion of food, in which large nutrient molecules (such as proteins, carbohydrates, and fats) are broken down into smaller molecules; the conservation and transformation of chemical energy; and the construction of cellular macromolecules from smaller precursors. Many inherited human diseases, such as albinism and phenylketonuria, result from a deficiency of a particular enzyme. Enzymes also have valuable industrial and medical applications. The fermenting of wine, leavening of bread, curdling of cheese, and brewing of beer have been practiced from earliest times, but not until the 19th century were these reactions understood to be the result of the catalytic activity of enzymes. Since then, enzymes have assumed an increasing importance in industrial processes that involve organic chemical reactions. The uses of enzymes in medicine include killing disease-causing microorganisms, promoting wound healing, and diagnosing certain diseases
Metabolism of Biomolecules
Before we study the details of metabolism, we must review the structure and functions(Chemistry) of
the four major classes of biomolecules.
Introduction
Overview of metabolism
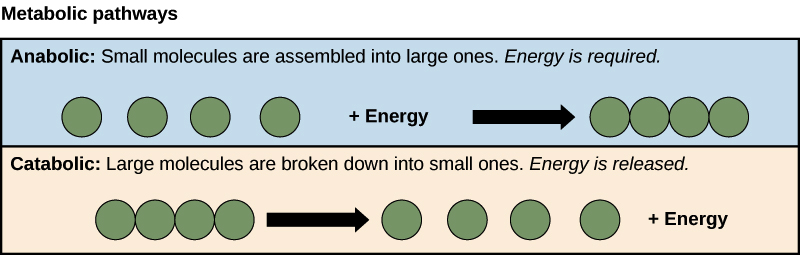
Anabolic and catabolic pathways
Nutrition
Nutrition is a science of food and its relationship to health. Nutrition refers to nourishment that
sustains life. The study of nutrient requirements and the diet providing these requirements is also
known as ‘nutrition’. It includes the uptake of food,
liberation of energy, elimination of wastes and all the processes of synthesis essential for
maintenance, growth and reproduction.. It is a relatively new science
with roots in physiology and biochemistry and is interrelated to various other subjects like
Medicine, Agriculture, Food science & technology, Biochemistry, Biological sciences,
Economics, Psychology, etc.
‘Balanced diet’ is defined as that ‘diet providing adequate nutritional needs as well as extra
allowance for stress from different foods belonging to different food groups in specific quantities
and proportions’. Since all foods don’t have similar nutritional quality, the nutrients provided
and thus the health depends on the choice & quantity of foods selected. For a healthy & active
life, diets should be planned on sound nutritional principals. Optimum nutrition /adequate
nutrition or good nutrition is a diet “that provides all dietary nutrients in respect of kind and
amount, and in proper state of combination or balance, so that the organism may always meet the
varied exogenous and endogenous stresses in life, whether in health or disease, with a minimal
demand or strain on the body’s natural homeostatic mechanisms”. Thus while
planning the diet, in addition to the calorific value, the quantity and quality of food(Vitamins & Minerals) is taken into
consideration. The three proximate principles of food are Protein, Carbohydrate and Fat. The
main energy food sources are carbohydrate and fats; whereas for growth and development
protein food sources are required. Deficiency, excess or imbalance of nutrients results in
malnutrition, which could be either under nutrition or overnutrition. Proper nutrition is required
for prevention of illness as well as for treatment of the illness.
Vitamins
Vitamins are organic compounds. Although they are required in small amounts, but are essential
for many important functions of the body. They can not be synthesized by the body. Due to
shortage of specific vitamins various deficiency diseases could occur. The vitamins were
designated by letters A, B and so on before their structures were determined. Foods contain small
quantities of these vitamins.
Minerals are required for many purposes like forming the frame and rigid structure of the body,
as part of the body/cell fluids and for number of cellular and sub cellular physiological functions.
Homeostasis
Homeostasis, any self-regulating process by which biological systems tend to maintain stability while adjusting to conditions that are optimal for survival. If homeostasis is successful, life continues; if unsuccessful, disaster or death ensues. The stability attained is actually a dynamic equilibrium, in which continuous change occurs yet relatively uniform conditions prevail.
Water is made up of 2 hydrogen atoms and 1 oxygen atom (Figure 3.1 “The Water Molecule”). A human body is made up of mostly water. An adult consists of about 37 to 42 liters of water, or about eighty pounds. Fortunately, humans have compartmentalized tissues; otherwise we might just look like a water balloon! Newborns are approximately 70 percent water. Adult males typically are composed of about 60 percent water and females are about 55 percent water. (This gender difference reflects the differences in body-fat content, since body fat is practically water-free.
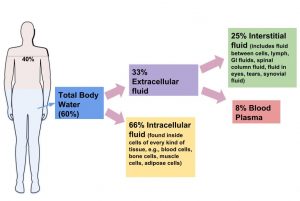
Fluid and Electrolyte Balance
Although water makes up the largest percentage of body volume, it is not actually pure water but rather a mixture of cells, proteins, glucose, lipoproteins, electrolytes, and other substances. Electrolytes are substances that, when dissolved in water, dissociate into charged ions. Positively charged electrolytes are called cations and negatively charged electrolytes are called anions. For example, in water sodium chloride (the chemical name for table salt) dissociates into sodium cations (Na+) and chloride anions (Cl−). Solutes refers to all dissolved substances in a fluid, which may be charged, such as sodium (Na+), or uncharged, such as glucose. In the human body, water and solutes are distributed into two compartments: inside cells, called intracellular, and outside cells, called extracellular. The extracellular water compartment is subdivided into the spaces between cells also known as interstitial, blood plasma, and other bodily fluids such as the cerebrospinal fluid which surrounds and protects the brain and spinal cord (Figure 3.2 “Distribution of Body Water”). The composition of solutes differs between the fluid compartments. For instance, more protein is inside cells than outside and more chloride anions exist outside of cells than inside.
ACID–BASE BALANCE
Maintenance of acid–base balance is fundamental for the normal functioning of biological processes, mainly due to the pH dependence of enzyme function. This article reviews definitions of acid–base balance and describes normal physiology of acid–base metabolism in the extracellular fluid and blood. The individual roles of the kidney, liver, bone, and lungs in maintenance of acid–base balance are outlined in detail in both health and disease. The pathogenesis of common conditions (diabetes, renal failure, drug intoxication) affecting acid–base balance are assessed as well as potential treatment strategies. The impact of dietary intake on acid–base status is also discussed.
Molecular Biology
The field of molecular biology has a profound impact in medical science investigation. Major advances in molecular biology over the last four decades have stimulated research and progress in almost all the disciplines of life science.
This driving force involves:
(1) the development of more and more sophisticated experimental techniques in molecular biology with a broad, interdisciplinary applicability;
(2) the ever-expanding flow of information of technical novelties and scientific discoveries across the scientific community; and
(3) the development of specific software and continuously updated databases for, respectively, analyzing and storing data on genotypes, gene expression levels, cytogenetic profiles and other molecular features.
This has changed the rationale and approach of scientific experimentations allowing revolutionizing discoveries not only in molecular biology but also in biochemistry, biophysics, biotechnology, cell biology, and genetics. One major example is the innovation in high-throughput biology, next generation sequencing and recombinant DNA technology, which made possible to unveil the high complexity of the genome and elucidate the precise mechanisms for the transmission of the genetic information. So, it is now proven that gene expression, DNA replication, DNA repair, and sister chromatid segregation are processes much more complicated than previously thought.
This complexity includes, but is not limited to:
(1) the existence of interconnected regulatory pathways involving also previously unexpected actors, such as non-coding RNAs;
(2) the relevance of epigenetic mechanisms and post-transcriptional modifications;
(3) the importance of the correct execution, timing, sequentiality and coordination of all cell cycle events;
(4) the pleiotropic functions of players operating in these processes; and
(5) the influence of the energetic metabolisms and environmental signals. Moreover, it has become evident that the deregulation of these molecular processes is associated with, and in certain circumstances is the direct cause of, a wide range of pathological conditions. Although this module is focused on life sciences, it is, however, necessary to mention the biomedical relevance of molecular biology-related investigations for drug discovery and the development of a more personalized medicine.
Given the rapidly changing and continuously evolving nature of the molecular biology field, we can anticipate that the revolutionary impact of molecular biology in medical sciences is only at the beginning and is far from being finished.
Organ Function Tests
This chapter provide a comprehensive view of vital organs with respect to their location, their importance and functions in the body; their respective disorders, diseases and various function tests.it provides basic knowledge on various routine function tests like Liver Function tests, Kidney Function tests, Thyroid Function tests, Adrenalin Function tests, Pancreas Function tests and Gastric Function tests. A few metabolic disorders have also been touched upon.
Biochemistry: Intra-departmental Moodle Training
This space will be a training "trial and error" playground for all faculty members to practise what they learn via the training videos. Any mistakes are reversible, so one need not worry about going ahead and exploring the software via this course.