Homeostasis
Homeostasis, any self-regulating process by which biological systems tend to maintain stability while adjusting to conditions that are optimal for survival. If homeostasis is successful, life continues; if unsuccessful, disaster or death ensues. The stability attained is actually a dynamic equilibrium, in which continuous change occurs yet relatively uniform conditions prevail.
Water is made up of 2 hydrogen atoms and 1 oxygen atom (Figure 3.1 “The Water Molecule”). A human body is made up of mostly water. An adult consists of about 37 to 42 liters of water, or about eighty pounds. Fortunately, humans have compartmentalized tissues; otherwise we might just look like a water balloon! Newborns are approximately 70 percent water. Adult males typically are composed of about 60 percent water and females are about 55 percent water. (This gender difference reflects the differences in body-fat content, since body fat is practically water-free.
Fluid and Electrolyte Balance
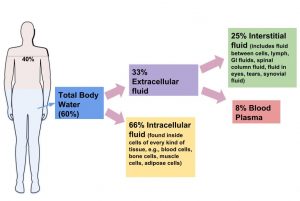
Although water makes up the largest percentage of body volume, it is not actually pure water but rather a mixture of cells, proteins, glucose, lipoproteins, electrolytes, and other substances. Electrolytes are substances that, when dissolved in water, dissociate into charged ions. Positively charged electrolytes are called cations and negatively charged electrolytes are called anions. For example, in water sodium chloride (the chemical name for table salt) dissociates into sodium cations (Na+) and chloride anions (Cl−). Solutes refers to all dissolved substances in a fluid, which may be charged, such as sodium (Na+), or uncharged, such as glucose. In the human body, water and solutes are distributed into two compartments: inside cells, called intracellular, and outside cells, called extracellular. The extracellular water compartment is subdivided into the spaces between cells also known as interstitial, blood plasma, and other bodily fluids such as the cerebrospinal fluid which surrounds and protects the brain and spinal cord (Figure 3.2 “Distribution of Body Water”). The composition of solutes differs between the fluid compartments. For instance, more protein is inside cells than outside and more chloride anions exist outside of cells than inside.
ACID–BASE BALANCE
Maintenance of acid–base balance is fundamental for the normal functioning of biological processes, mainly due to the pH dependence of enzyme function. This article reviews definitions of acid–base balance and describes normal physiology of acid–base metabolism in the extracellular fluid and blood. The individual roles of the kidney, liver, bone, and lungs in maintenance of acid–base balance are outlined in detail in both health and disease. The pathogenesis of common conditions (diabetes, renal failure, drug intoxication) affecting acid–base balance are assessed as well as potential treatment strategies. The impact of dietary intake on acid–base status is also discussed.
